Molar Mass of Oxalic Acid
A place for chemistry enthusiasts and professionals alike. It forms a variety of salts for example sodium oxalate Na 2 C 2 O 4 and several esters such as dimethyl oxalate C 2 O 4 CH 3 2It is a conjugate base of oxalic acidAt neutral pH in aqueous solution oxalic acid converts completely to.

Equivalent Weight Of Crystalline Oxalic Acid Is Youtube
A mixture of formic acid and oxalic acid is heated with conc.
. NaOH HOOC-C6H4-COOK Æ NaOOC-C6H4-COOK H2O By measuring the volume of the 02M NaOH solution dispensed from the buret that is necessary to react completely with a weighed sample of KHP the exact concentration of NaOH solution is calculated. Molar mass of NaOH is the sum total of individual elements molar mass 23161 40gmol. Density of inorganic substances.
The strength of an acid can be determined using a standard solution of a base. For example we could weigh out exactly 01 moles of primary standard oxalic acid and dilute it to one liter in a volumetric flask to make a 01000 molar solution. Chemistry tools periodic table unit converters and more.
Density of Aqueous Solutions of some Inorganic Substances - Changes in density of aqueous solutions with changes in concentration at 20C. The value of x y is. Both titrations involve in the neutralization reaction of an alkali.
It occurs naturally including in some foods. Ethanedioate is an anion with the formula C 2 O 4 2This dianion is colorless. The reaction between NaOH and KHP molar mass 20423 gmole is as follows.
Buy Nitric Acid Products Online Here Or By Phone. Density of acetic acid citric acid formic acid D-lactic acid oxalic acid and trichloroacetic acid in water is plotted as function of wt molkg water and moll solution. Pure formic acid is a liquid with a flash point of 69 C much higher than that of gasoline 40 C or ethanol 13 C.
The gas produced is collected and treated with OH solution whereby the volume decreases by 6 1 th. A Fractionation using sulfuric acid in dioxane 13 wt birch wood 7 wt glyoxylic acid 3 wt H 2 SO 4 3 wt water and 74 wt dioxane at 60 C for 48 h. The mass of oxalic acid H2C2O4aq and Equation 4 are then used to determine the number of moles of potassium hydroxide present.
H 2 S O 4. Formic acid contains 53 gL hydrogen at room temperature and atmospheric pressure which is three and a half times as much as compressed hydrogen gas can attain at 350 bar pressure 147 gL. 0486 g H2C2O4 mol H 2C O4 90036 g H2C2O4 2 mol KOH 1 mol H2C2O4 00108 mol KOH 6 Finally the molarity for potassium hydroxide is calculated as follows.
B Fractionation using sulfuric acid. This process is called acidimetry. In the same way the strength of a base can be found with the help of a standard solution of an acid which is known as alkalimetry.
From this result we can. Main standards need not necessarily be the standard solutions used in the acid-base titration. The molar ratio of the two acids formic acidoxalic acid is x.
Molarity of KOH KOH 001080 mol KOH 001798L KOH 06007 M. The number of moles present can be reliably estimated from the measured weight and the known molar mass of a primary standard such as oxalic acid which can be exactly weighed out in the pure form. Concentrated nitric acid 68 - 70 is a transparent colorless or yellowish fuming suffocating hygroscopic corrosive liquidThis chemical attacks almost all metals.
A primary standard is any material that can be exactly weighed out in pure form such as oxalic acid so that the number of moles present can be estimated reliably by the measured weight and the known molar mass. Molegiven massmolar mass from this basic formula given mass molemolar mass.

Equivalent Weight Of Crystalline Oxalic Acid Is Youtube
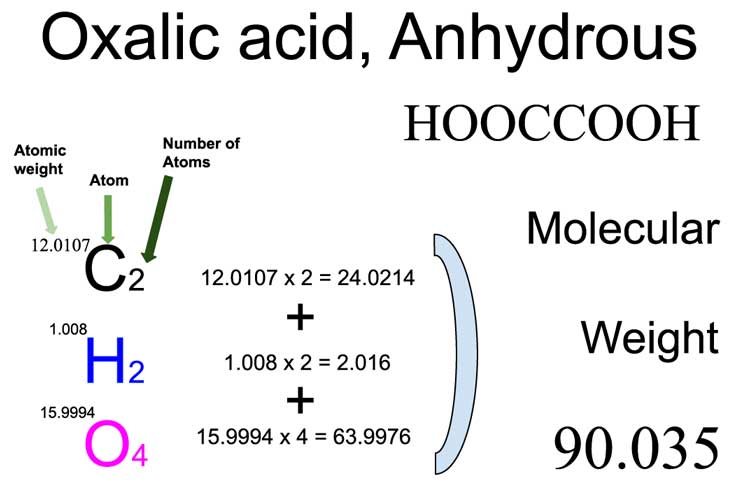
Oxalic Acid Hooccooh Molecular Weight Calculation Laboratory Notes
What Is The Molecular Mass Of Hydrated Oxalic Acid Quora

Solved Calculate The Molar Mass Of Oxalic Acid Dihydrate Chegg Com

The Mass Of Oxalic Acid Crystals H 2 C 2 O 4 2h 2 O Required To Prepare 50 Ml Of A 0 2 N Youtube
Comments
Post a Comment